Advocacy
Recent State & National Policy Efforts
- USPSTF – May 2021
- Updated CRC screening guidelines
- Lowered screening age to 45 for patients at average risk of developing CRC
- Removed “diagnostic” from clinically mandated secondary screening colonoscopies
- Updated CRC screening guidelines
- CA AB 342 (Gibson) – October 1, 2021
- Removes cost sharing in commercially insured patients for secondary colonoscopies following abnormal primary test
- Centers for Medicare & Medicaid Services (CMS) – December 2021
- Addition of CRC Quality Measure to Medicaid/Medi-Cal Adult Core Set
CMS CRC screening reporting requirements
C4 Efforts – Highlighting the Advocacy Processes
- Removal of “Diagnostic” from Screening Colonoscopies
The 2016 United States Preventive Services Task Force (USPSTF) CRC screening recommendations stated that the follow up colonoscopy after a positive fecal test was a diagnostic colonoscopy. Because of this wording in the 2016 guideline, insurance companies were charging cost sharing for the colonoscopy after a positive non-colonoscopic screening test.
In 2020, when comments were allowed concerning revisions of the 2016 recommendations, C4 and numerous collaborators requested that the colonoscopy after a positive non-colonoscopic screening test was a required secondary part of the screening process. The USPSTF accepted the changes and updated their recommendations to the following:
Positive results on stool-based screening tests require follow-up with colonoscopy for the screening benefits to be achieved. They included that screening strategies may include combinations of screening tests. - Implications for Medicare Patients – Expanded Coverage of Screening Colonoscopies
Nationally the change in the USPSTF guidelines resulted in the Departments that regulate commercial insurance issuing a regulation in January of 2022 that removed cost sharing in the CRC screening process for all patients commercially insured in the US. In addition, CMS Medicare recently proposed that in 2023, cost sharing be removed from the CRC screening process for all patients insured by Medicare. - Passage of CA AB342 (2021) – Elimination of Surprise Billing for Screening Colonoscopies in Commercially-insured Patients
The USPSTF update CRC screening recommendation (2021) allowed C4 and ASC/CAN to co-sponsor a bill Gipson AB 342 (2021) that would remove cost sharing in the CRC screening process for commercially insured Californians. This bill passed and was signed into law by Governor Newsom in October 2021. - Adding colorectal cancer screening to CMS Medicaid Adult Core Set of Quality Measures
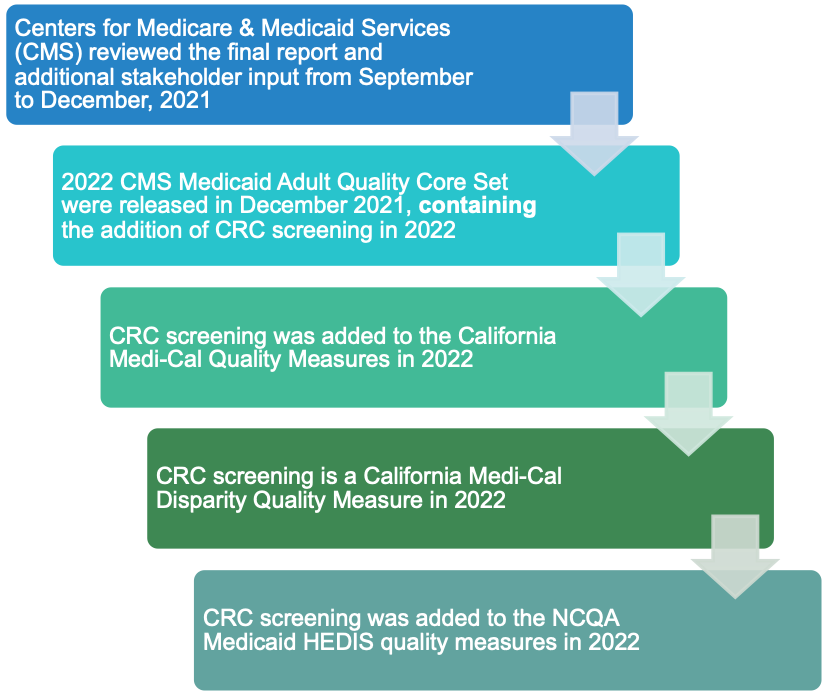
C4 provided national leadership for adding CRC screening to the Centers for Medicare and Medicaid (CMS) Adult Core Set of Quality Measures.
The rationale for accomplishing this is outlined in the following paper: Hitchcock ME, Green BB, Anderson DS. Advancing Health Equity for Medicaid Beneficiaries by Adding Colorectal Cancer Screening to the Centers for Medicare and Medicaid Services Adult Core Set. Gastroenterology. Published online December 20, 2021. doi:10.1053/j.gastro.2021.12.25
The process was further outlined in the following blog written by Drs. Anderson and Hitchcock in collaboration with FightCRC advocacy partners and highlighted here: https://fightcolorectalcancer.org/blog/increasing-access-to-colorectal-cancer-screening/
C4 worked with national partners, including the National Association of Chronic Disease Directors and Leavitt Partners, FightCRC, and NCCRT to add colorectal cancer screening to the CMS Medicaid Adult Core Set of Quality Measures. This process required developing focus responses to the CMS/Mathematica criteria for consideration and addition of new quality measures. In addition, we recruited two CMS voting members to introduce the recommendations in January 2021, and we mobilized national support by stakeholders to encourage adoption of the new quality measure to help to reduce inequities in care delivery for Medicaid patients, as reflected in the lower screening rates for this patient population. CMS noted during the May 2021 voting period that this was the first time, ever, that addition of a quality measure received unanimous support. CMS released their final recommendations in December 2021, which included addition of the CRC screening quality measure for Medicaid patients.
